
Ocean acidification is a term used to describe a major shift in the chemistry of the ocean caused by sharp increases in carbon dioxide in the global atmosphere. A portion of this carbon dioxide is absorbed by ocean waters. This does not mean that the ocean is turning into an "acid." It does however mean that the ocean is becoming more acidic—in other words the pH level of seawater is becoming lower and moving closer to the acid end of the scale.
Ocean acidification happens when carbon dioxide reacts with water to form carbonic acid. The rate of ocean acidification has increased as humans continue to release rampant levels of carbon dioxide through the burning of fossil fuels like oil, coal, and natural gas. In spring of 2013, the atmospheric concentration of carbon dioxide reached 400 parts per million, higher than the earth had seen in more than 800,000 years. In 2021 we passed 420 parts per million. Since people started burning fossil fuels during the Industrial Revolution, the global average pH of the surface ocean has decreased by 0.11 pH units, which is a 30% increase in acidity. If carbon emissions are not reduced, by the end of the century ocean pH is predicted to be lower than the earth has seen in 20 million years.
What does this mean? The ocean acts as a carbon "sink," buffering our atmosphere from what would otherwise be much higher concentrations of carbon dioxide. While this phenomenon means we're experiencing lower atmospheric CO2 than we would without the ocean, the consequence is the increase in ocean acidity. The rate of change in ocean pH is so much greater than what the earth has historically seen, some marine organisms will not adapt to their new environment. Calcifying organisms, such as shellfish and corals, are particularly vulnerable to ocean acidification, which impedes the ability of these organisms to produce and maintain their shells. Some negative impacts have already been documented, such as farmed oyster larva in the Pacific Northwest unable to form shells during low pH events. Laboratory experiments show that elevated carbon dioxide alters clownfish behavior, making them potentially more vulnerable to predators. Coral reefs, already impacted by rising ocean temperatures, are greatly threatened by ocean acidification. This infographic summarizes what is known about the impact of ocean acidification on specific California marine species.
How do we address this problem? The ultimate answer is to stop burning fossil fuels. Get involved in your community to support decisions that help fight ocean acidification. Talk to your family, neighbors, and elected officials about ways we can reduce fossil fuel use on a community or government level and how we can make it easier for individuals to consume less. (In 2018, the State of California passed the 100 Percent Clean Energy Act, calling for 100 percent of total retail sales of electricity in California to come from eligible renewable energy and zero-carbon resources before 2046.) On a personal level, commit to conserving energy wherever possible. Walk, bike, ride public transit, and carpool instead of driving alone. Reduce your waste by thinking before you buy something. Conserve electricity, gas, and water (which takes energy to heat, pump, and treat) at home and work. Eat less meat, since livestock farming produces high amounts of greenhouse gases. And help the ocean cope by reducing other pressures on marine organisms by consuming only seafood that has been fished or farmed sustainably, picking up trash in your neighborhood and at the beach, practicing smart use of pesticides and fertilizers, and cleaning up after pets. Research is ongoing to study how marine organisms such as seagrass and kelp locally reduce dissolved CO2, so protecting and restoring these coastal habitats may be an important tool in dealing with ocean acidification.

This comic was co-produced by Years of Living Dangerously, a SHOWTIME climate change documentary series, and Symbolia, a magazine where "comics and journalism meet." Find more information at symboliamag.com and yearsoflivingdangerously.com.
Text Content of the comic:
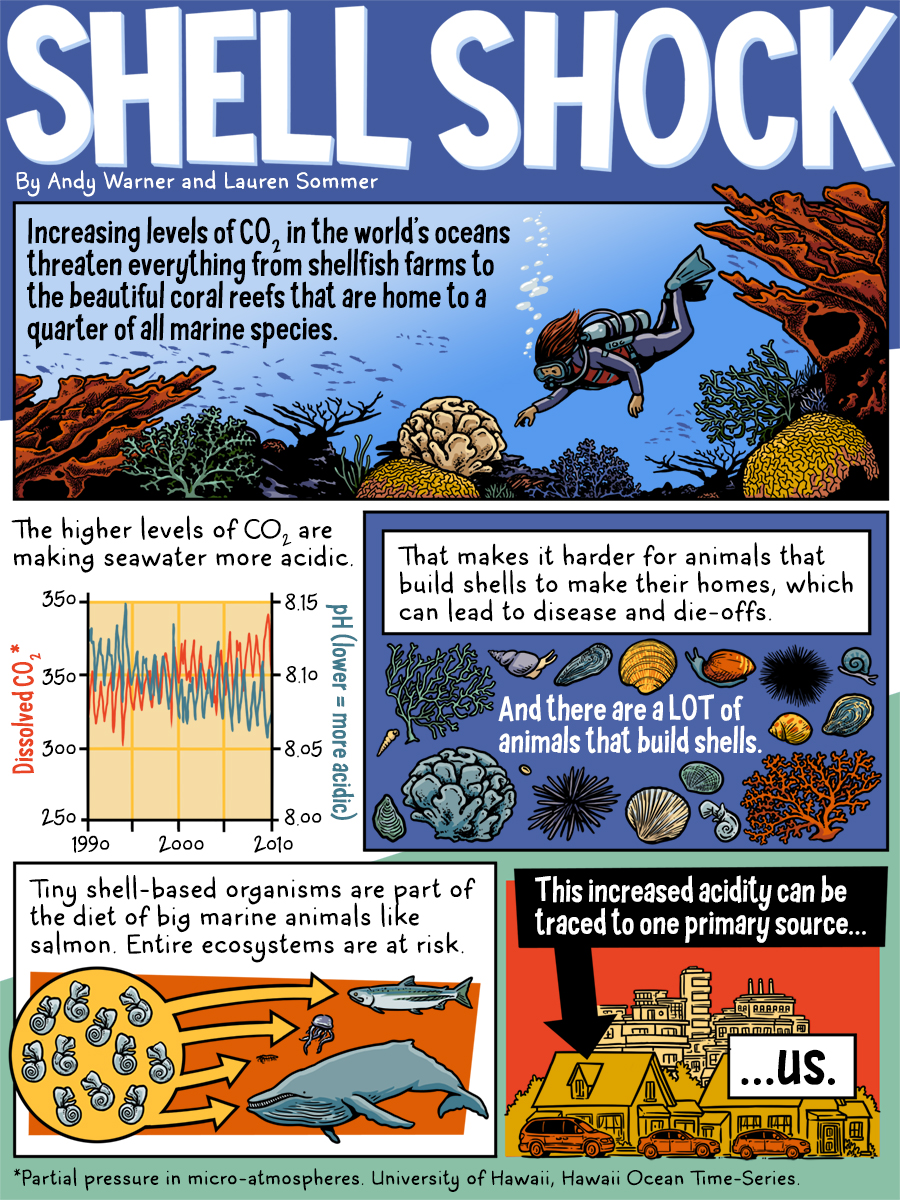
"SHELL SHOCK" by Andy Warner and Lauren Sommer
Increasing levels of CO2 in the world's oceans threaten everything from shellfish farms to the beautiful coral reefs that are home to a quarter of all marine species.
The higher levels of CO2 are making seawater more acidic. That makes it harder for animals that build shells to make their homes, which can lead to disease and die-offs. And there are a LOT of animals that build shells.
Tiny shell-based organisms are part of the diet of big marine animals like salmon. Entire ecosystems are at risk. This increased acidity can be traced to one primary source...us.
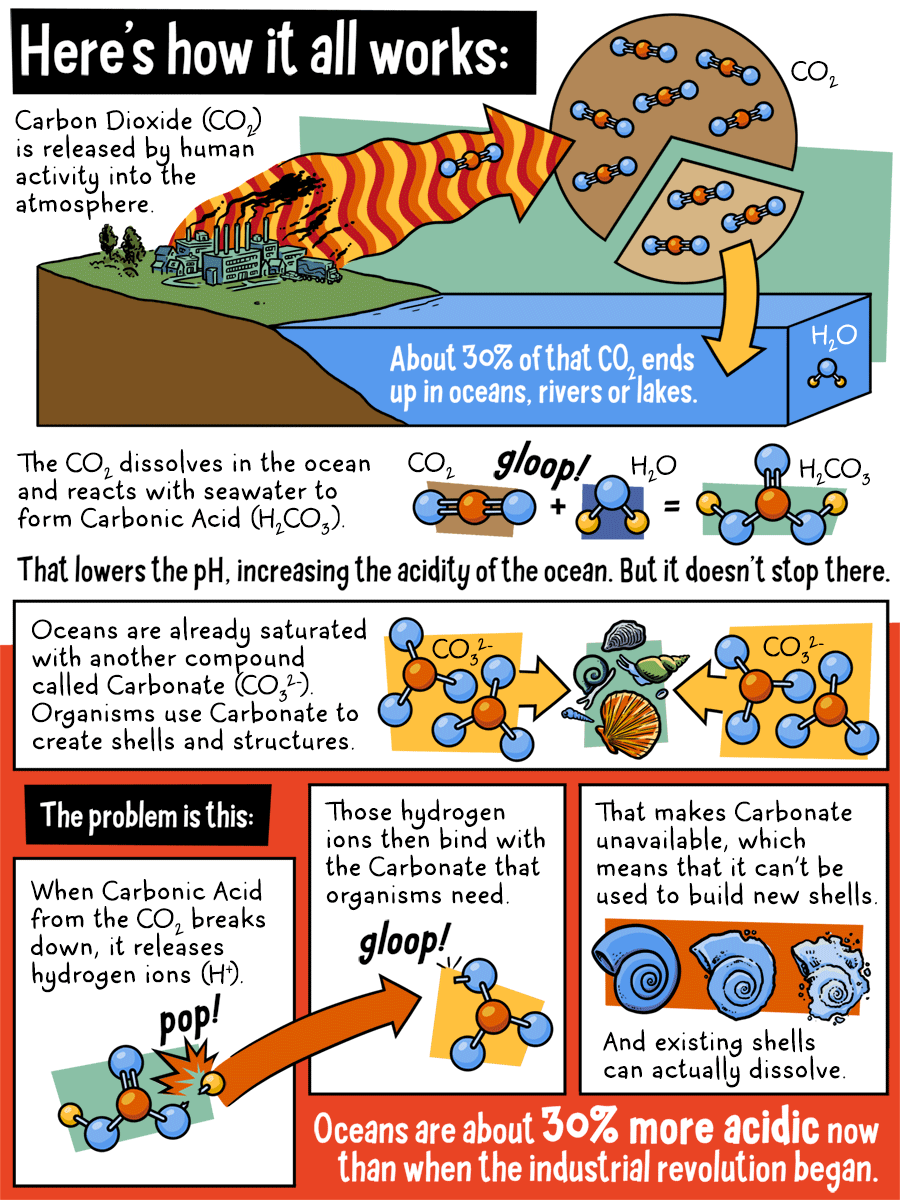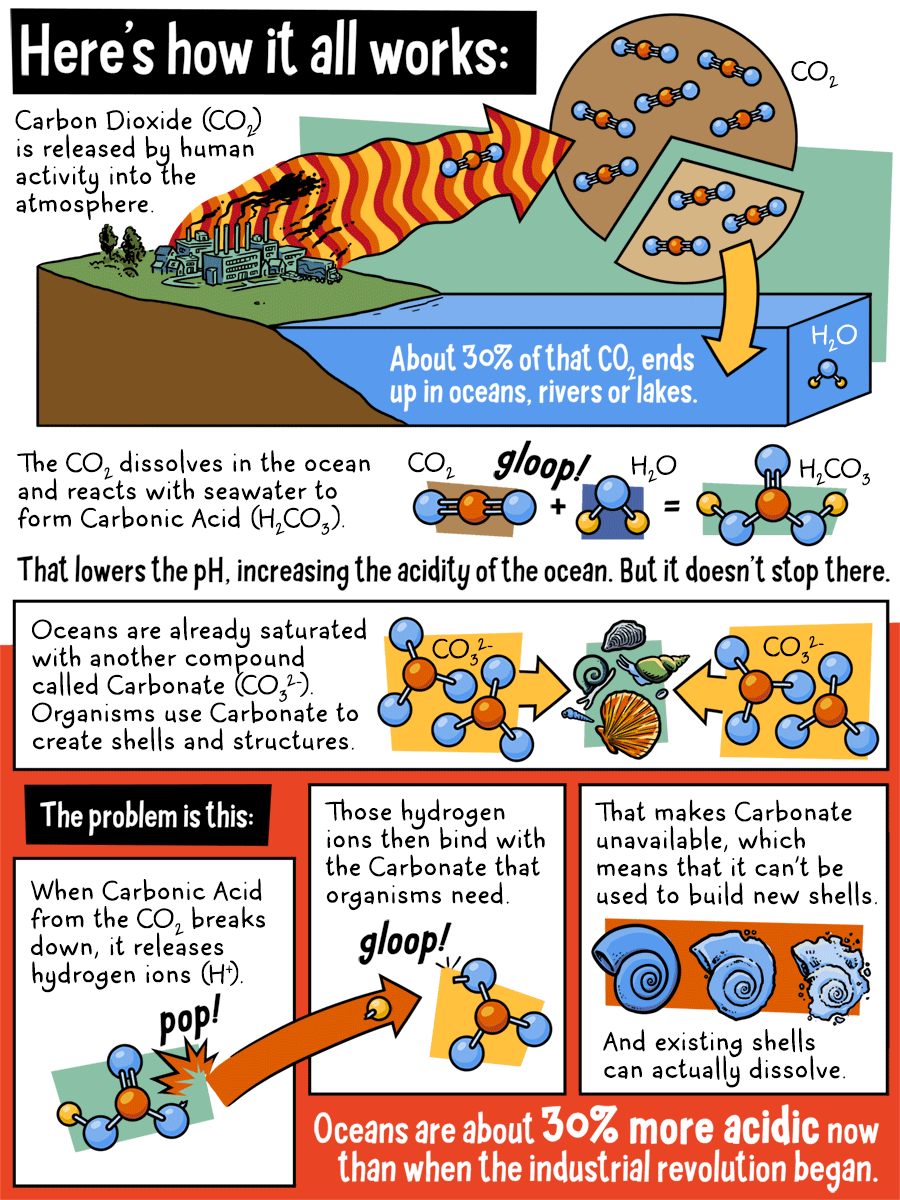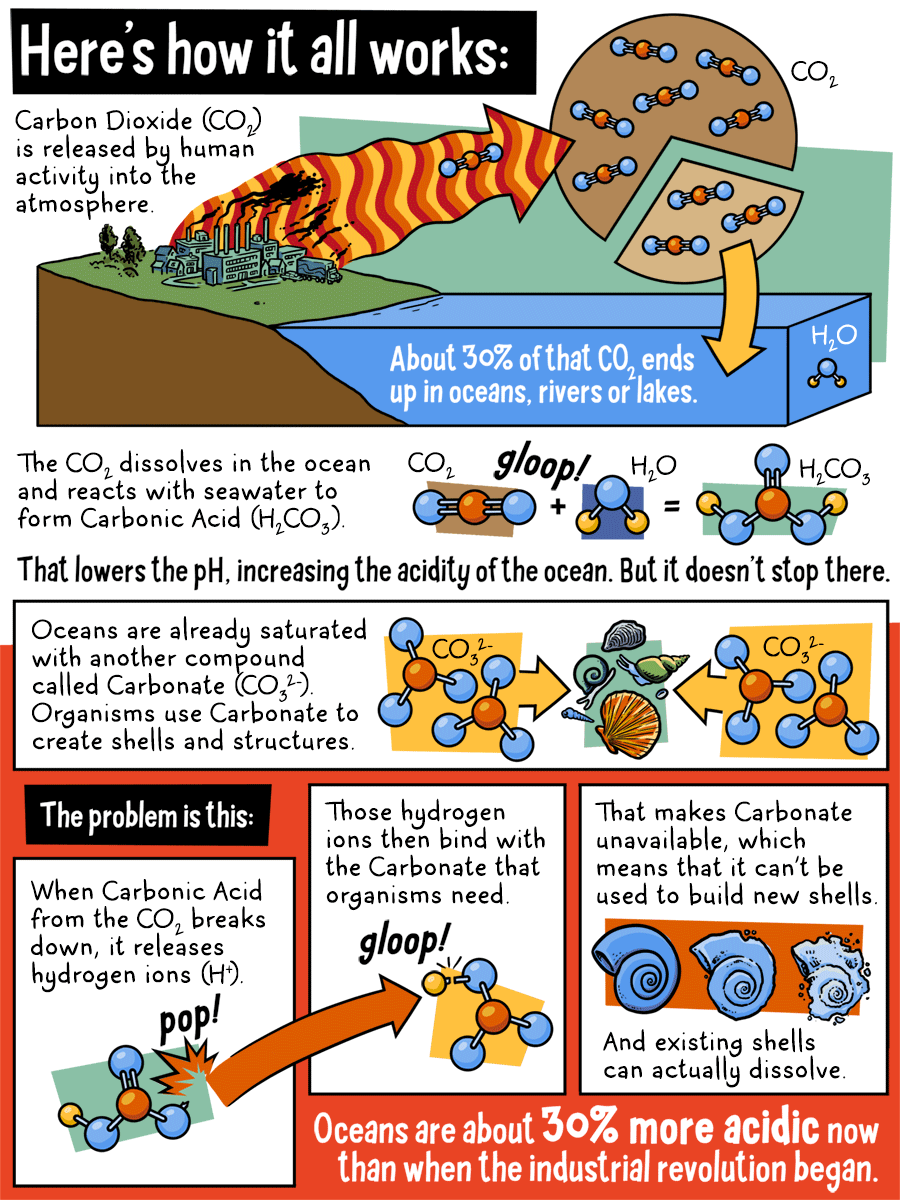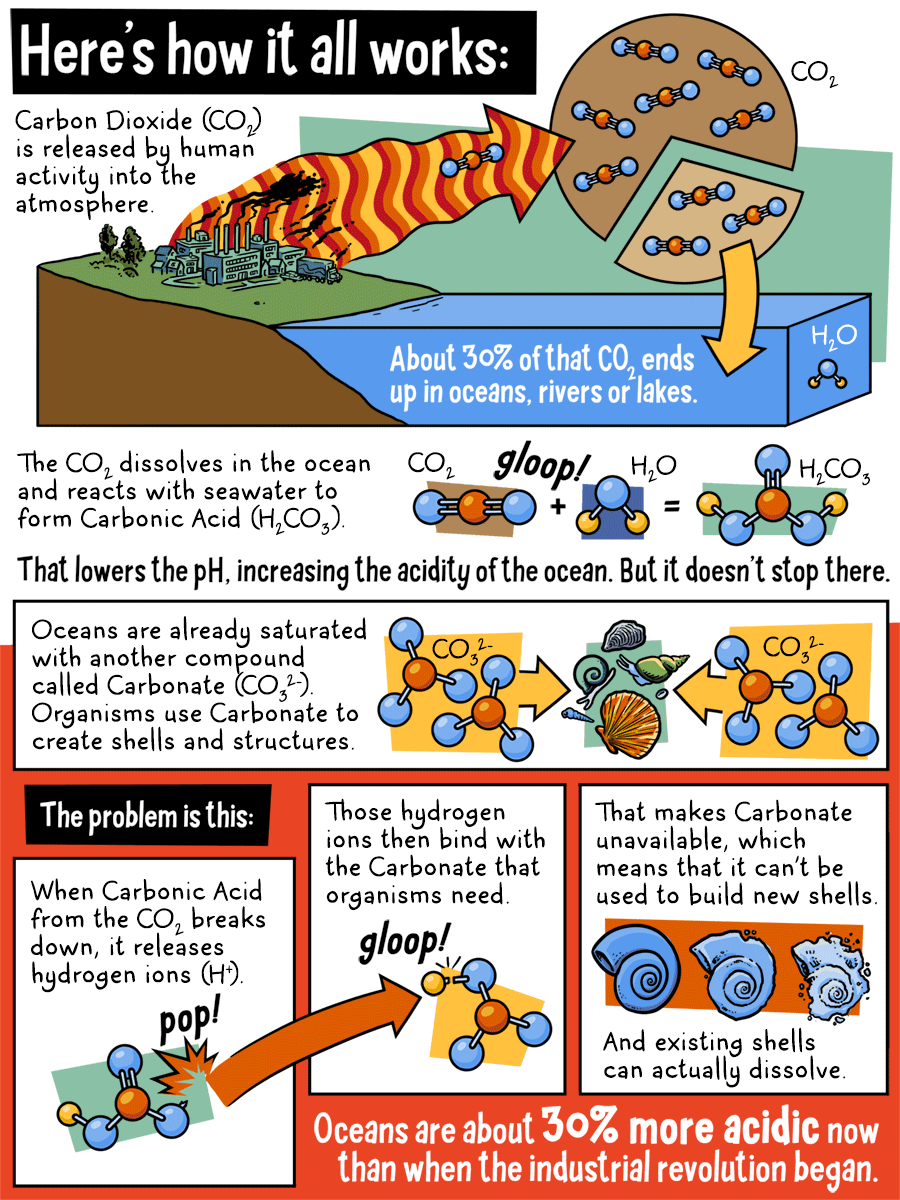
Here's how it all works:
Carbon Dioxide (CO2) is released by human activity in to the atmosphere. About 30% of that CO2 ends up in oceans, rivers, or lakes. The CO2 dissolves in the ocean and reacts with seawater to form Carbonic Acid. That lowers the pH, increasing the acidity of the ocean. But it doesn't stop there.
Oceans are already saturated with another compound called Carbonate. Organisms use Carbonate to create shells and structures.
The problem is this:
When Carbonic Acid from the CO2 breaks down, it release hydrogen ions. Those hydrogen ions then bind with the Carbonate that organisms need. That makes Carbonate unavailable, which means that it can't be used to build new shells. And existing shells can actually dissolve. Oceans are about 30% more acidic now than when the industrial revolution began.
The increasing acidity of the oceans is already having a human impact. "There's a perfect storm of events. Changes are happening much too quickly," says Terry Sawyer, co-owner of Hog Island Oyster Company in California. A few years ago the hatcheries in Oregon and Washington which supply him with baby oysters began seeing huge tanks full of larvae die off. The hatcheries were filling their tanks with sea water. That turned out to be a problem. The increased acidity of the ocean, combined with a natural upwelling of low pH water off the Pacific coast devastated the young oysters. Just like the oysters he grows, Terry Sawyer says he'll have to adapt to the rapidly changing environment of the oceans. "We don't have the luxury to look the other way. Our challenge is to figure out where we can grow, what species we can grow, what adaptations we can implement. We are having an effect on the oceans. We've got to start doing something about it."
From Symbolia, Years of Living Dangerously